Whats the Deal with Hydroxychloroquine? The current state of scientific knowledge.
Follow @PapersCovidLet’s take a quick look at what the science says today about Hydroxychloroquine or HCQ.
A recent letter in the Annals of Internal Medicine offers a TL;DR
Data to support the use of HCQ and CQ for COVID-19 are limited and inconclusive.
The French Study
If you’ve heard anything at all about HCQ, it’s probably in some way related to this French study which came out without peer review in mid March. You know, the one that produced this graph that was making the rounds a couple weeks ago.
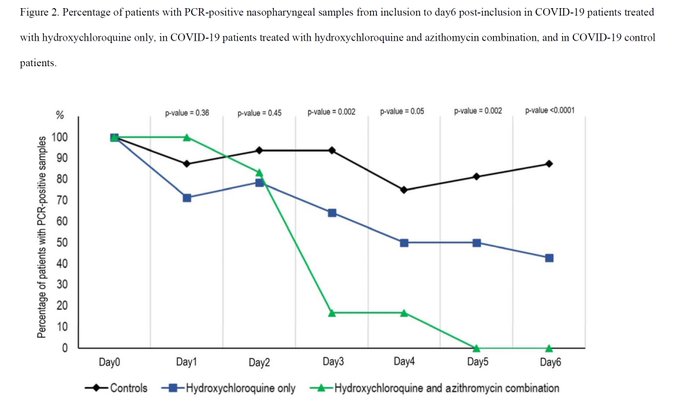
One of the biggest problems with this study was that 6 of the 23 patients receiving HCQ in the study were not included in the final results.
A letter to the BMJ summarizes the problem well and notes how outcomes were actually nominally better for the people not taking HCQ in the French study.
The reasons for non-completion were that one patient died, three were transferred to the intensive care unit (ICU), and two withdrew. None of the 16 patients in the control group died, withdrew, or needed care in an ICU.
The authors also deviated from their self-registered study protocol. The published study had 2 groups, control and treatment, whereas the pre-registered protocol called for 3 groups. Likewise, the authors had proposed to measure the outcome of the study by looking at viral load on days 1, 4, 7, and 14 but only did so on day 6 in the final study.
An additional issue
19/
— Rahul Ganatra (@rbganatra) March 29, 2020
Finally, the primary endpoint is a surrogate endpoint, and none of the clinical secondary endpoints specified are reported. This is called selective outcome reporting, or outcome reporting bias.
And the bottom line
You can see the whole thread here21/
— Rahul Ganatra (@rbganatra) March 29, 2020
Bottom line:
This study is at high risk of bias. Due to small size, lack of randomization or blinding, and multiple sources of bias towards a benefit of HCQ, this evidence should be considered low quality. Better-designed RCTs are needed (and are in process).
(End)
In-vitro and theoretical evidence in favor
Chloroquine was studied in an artificial environment (think petri-dish). The gist of this kind of study is they put some drugs into a cell infected with the virus. At a high enough concentration the drug might inhibit the virus, the cell, or both. Ideally you want to find a drug that inhibits the virus without killing or damaging the cell. In the image below the x axis are different concentrations of the drug. The y axis for pink is the degree of inhibition of the virus, while the y axis for blue is the toxicity to the cell.
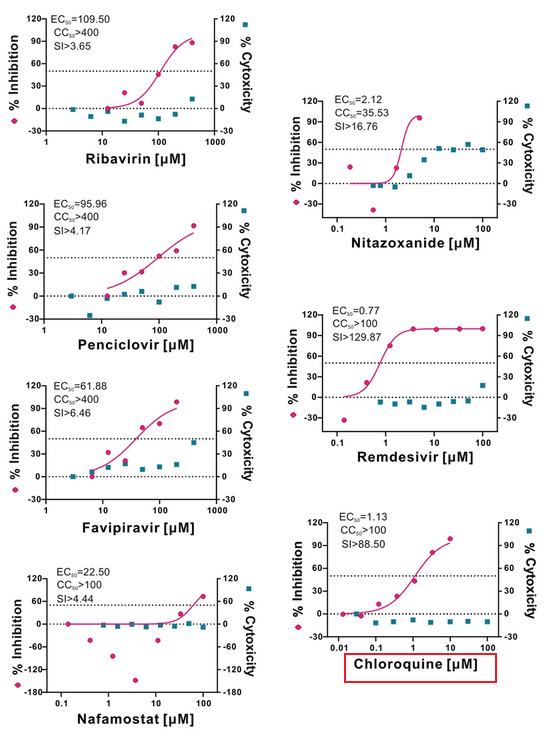
HCQ and Remdesivir were promising in this study because they were able to achieve a high degree of inhibition at relatively tolerable concentrations. The SI number given for each compound, or selectivity index, quantifies the extent to which inhibition and toxicity are trading off in the experiment.
There are also some theoretical reasons why we might be helpful for Chloroquine and Hydroxychloroquine. See the paper we’ve just been talking about for more information.
Recent Studies
An earlier letter from physicians in China to BioScience Trends said the following about usage there:
Thus far, results from more than 100 patients have demonstrated that chloroquine phosphate is superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virusnegative conversion, and shortening the disease course according to the news briefing.
Another French study–the most recent–with different researchers was recently submitted as a preprint, but the title of the publication is also a good summary: No Evidence of Rapid Antiviral Clearance or Clinical Benefit with the Combination of Hydroxychloroquine and Azithromycin in Patients with Severe COVID-19 Infection.
This study looked at treating 7 men and 4 women with the same medication as in the prior French study. Highlights below:
- They used the same non randomized design as the prior study
- One patient had to discontinue due to developing the cardiac symptoms associated with HCQ
- 8 out of the remaining 10 were still positive 5-6 days after treatment started
A number of clinical trials are currently under way, but until these are finished, we may be stuck with the unsatisfying quote we began with. The evidence that exists is indeed “limited and inconclusive.”
UPDATE: The journal that published the original French study has issued a “a statement of concern”
does not meet the [International Society of Antimicrobial Chemotherapy’s] expected standard, especially relating to the lack of better explanations of the inclusion criteria and the triage of patients to ensure patient safety.