Next stop, phase 3 clinical trials: "hopeful" results from Remdesivir study released today
Follow @PapersCovidJust published in the NEJM.
Big for #COVID19 therapy: the compassionate use results for remdesivir in 53 patients looks very encouraging, especially in very sick patients on mechanical ventilation with 18% fatality (only, expect > 50%) and overall 68% improvement https://t.co/oP8eDK6jYL @NEJM pic.twitter.com/9kisPxy1un
— Eric Topol (@EricTopol) April 10, 2020
Key findings
- 53 patients (initially 61, 1 excluded to a dosing error and 7 with no post-treatment data)
- 17 of 30 patients on ventilation were extubated
- 3 or 4 patients on ECMO stopped receiving it
- “clinical improvement was observed in 36 of 53 patients (68%)”
- 25 of 53 were discharged
- 7 of 53 died
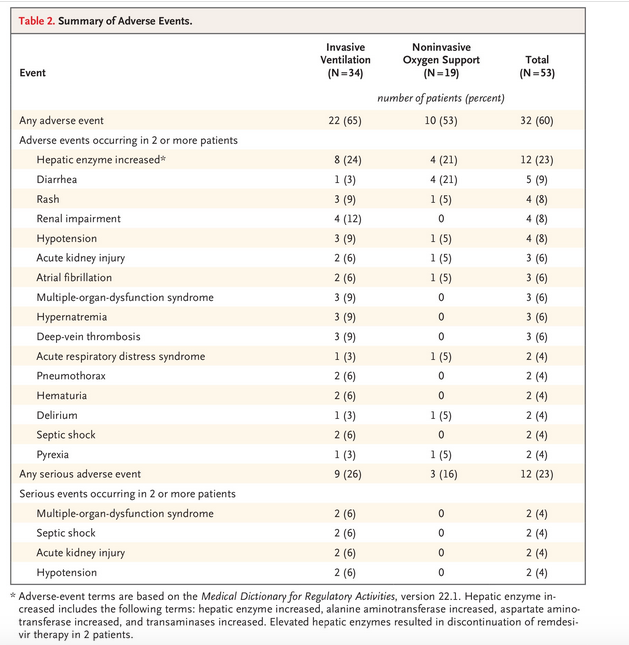
- 23% had one or more serious adverse events
Study participants were given a 10-day course of Remdesivir: an IV loading dose of 200mg and 100mg daily the following 9 days.
Follow-up was done from between 13 and 23 days after initiation of treatment (median 18 days) with the following results
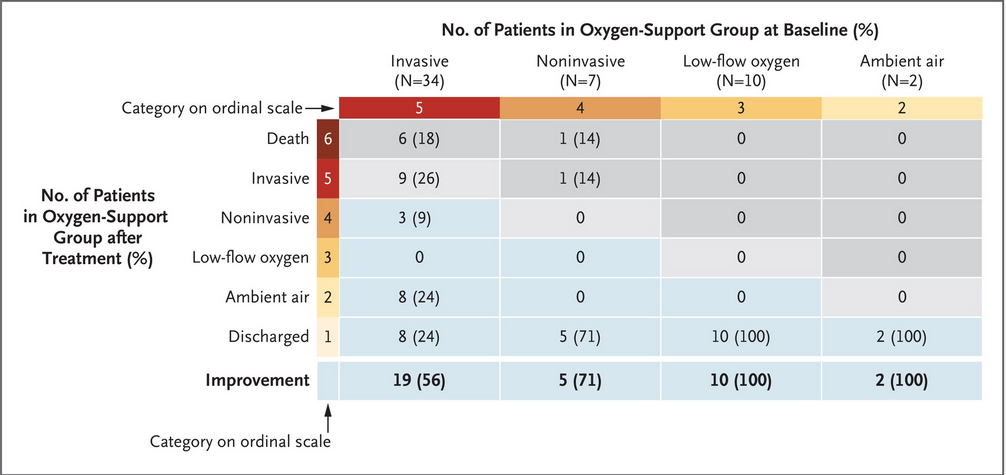
The way to read this is the columns track where people were initially and the rows are where they were after follow up. So for example, 6 of 34 patients (18%) were on invasive ventilaion but ultimately died; 5 of 7 (71%) were on noninvasive ventilation and woud up discharged, etc. In general a dark grey box was a worsening of codition, a light blue improvement, light grey stayed th same in terms of oxygen support. Overall, 68% improved while 15% worsened in the time between treatment initiation and follow up.
Though there is no conrol group here, Eric Topol writes of the study
The previous reports of patients w/ #COVID19 who required intubation, on a ventilator, all have fatality rates >50%. That’s historical controls from China, Italy, and the UK and go up to 93% fatality. We need more data for remdesivir but this really a good signal for efficacy.
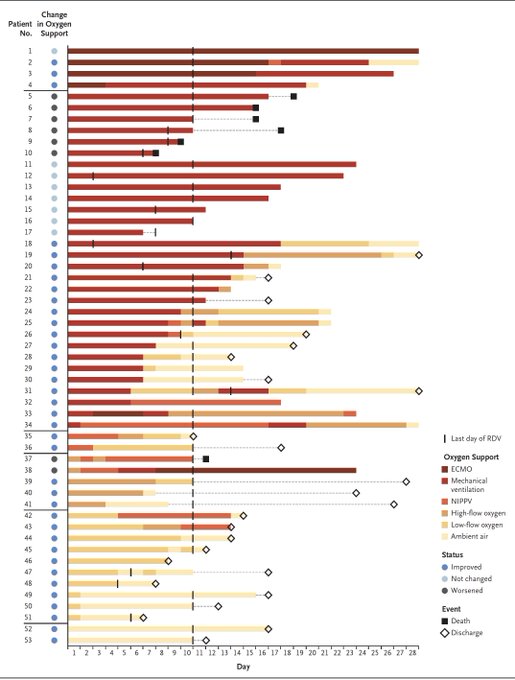
Another way of viewing the data, each row is a patient, the colors are the status of respiratory support
The baseline characteristics of the participants recruited from the US and 8 other countries
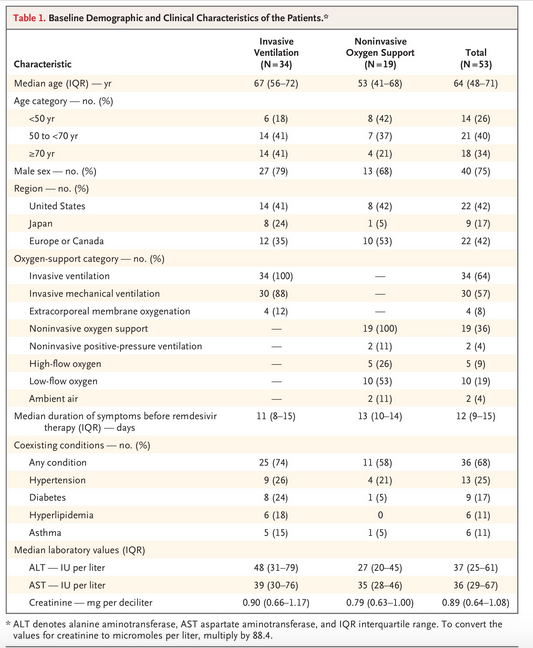
Note that 64% were receiving mechanical ventilation and 8% receiving ECMO.
Data on adverse events
The Washington Post spoke with the leader of the study
“We cannot draw definitive conclusions from these data, but the observations from this group of hospitalized patients who received remdesivir are hopeful,” said Dr. Jonathan D. Grein, Director of Hospital Epidemiology, Cedars-Sinai Medical Center, Los Angeles, and lead author of the journal article. “We look forward to the results of controlled clinical trials to potentially validate these findings.”
Up, next a controlled phase 3 study, most likely.
Here’s what others are saying
Encouraging data for compassionate use of #Remdesivir in #coronavirus patients. 57% of those treated with extubated which compares favorably with 20% rates historically. RCTs ongoing as needed to characterize efficacy https://t.co/6DJ6Qdguk0 pic.twitter.com/YNNKA3eWS8
— C. Michael Gibson MD (@CMichaelGibson) April 10, 2020
Interesting data on compassionate use Remdesivir in severe #COVID19 https://t.co/emNbrG4tVC
— Anand Swaminathan (@EMSwami) April 10, 2020
Caution: not for all comers
- Small study w/ no comparator
- 60% w/ adverse events, 23% serious
- 68% w/ decr O2 need 18 days - was this drug or time?
Authors w/ excellent conclusions pic.twitter.com/qjg1eyxLPq
Compassionate #remdisivir use (n=53) encouraging but doesn’t prove benefit in #COVID19.
— Kira Newman, MD, PhD (@KiraNewmanMDPhD) April 10, 2020
•Use criteria: O2 sat<94%, Cr clearance>30, LFTs<4x ULN
•No comparison arm
•13% mortality, 25% discharged, the rest still admitted (median f/u 18 days)
•Safety okhttps://t.co/dVUBSQ0fYh
Remdesivir in 61 COVID-19 patients compassionate use in NEJM. Report on 53 analyzed. Tough to make any conclusions - need outcomes from the randomized controlled trial. @DukeHealth @camwolfe pic.twitter.com/FWZ7iO3GwX
— Manesh Patel (@manesh_patelMD) April 10, 2020
The next thing to look out for are results from one of the randomized controlled trials underway.
Update 4/10/20 Gilead has made some changes to their study protocol for this drug.
Gilead has revised their RCT of Remdesivir in people with severe #COVID19: Initially it was n=400 now it’s n=2400, also Changed 1° endpoint from improvement in SpO2 and Fv to change in ordinal scale. Is it ok revise an ongoing trial in the midst of a pandemic with changing info? pic.twitter.com/DIGDUtYT8X
— Nick Mark MD (@nickmmark) April 10, 2020
One reason they might do this is because they fear the effect of the drug might be too small to be sigificant without a larger sample size as this person points out.
Omg, a 6x increase in sample size PLUS the power boost of 7 ordinal scale. Folks hyping todays uncontrolled, selection bias, inconclusive NEJM results should maybe stay quiet and await the RCT.
— Vinay Prasad (@VPrasadMDMPH) April 10, 2020
Page a stats colleague for what they think these changes mean for effect size https://t.co/LKAgIzeBOi